Abstract
Hypomethylating agent (HMA) plus venetoclax (VEN) regimens are standard of care in patients with acute myeloid leukemia (AML) ineligible for intensive chemotherapy. While the VEN label recommends continuous 28-day cycles, shortened VEN durations may induce similar response rates and improve tolerability. It is unknown how a VEN exposure reduced to 7 days during cycles compares to standard HMA + VEN. We retrospectively compared newly diagnosed AML patients treated with azacitidine (AZA) x 7 days plus VEN x 7 days (“7 + 7” regimen) from the first cycle (n = 82) vs patients treated with standard dose HMA + VEN (std-HMA/VEN) (n = 166). Composite complete remission rate was similar between cohorts (72% vs 72%; p = 0.95) and a median number of cycles to best response was 2 with “7 + 7” vs 1 with std-HMA/VEN (p = 0.03). Concerning toxicity, platelet transfusion rates during cycle 1 as well as early mortality at 8-weeks (6% vs 16%; p = 0.03) were lower in “7 + 7” cohort. Finally, the median OS was 11.2 months (2-year 28%) with “7 + 7” vs 10.3 months (2-year 34%) with “std-HMA/VEN” (p = 0.75). In summary, acknowledging limitations of a retrospective comparison, a shortened course of VEN used for 7 days every 28 days resulted in similar response rates and survival when compared to standard VEN exposure.

Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) represents a heterogeneous clonal disorder of myeloid precursors primarily affecting elderly individuals with an unfavorable prognosis [1]. Combination of the hypomethylating agent (HMA) azacitidine (AZA) with venetoclax (VEN), a B-cell lymphoma 2 (BCL2) inhibitor, is now approved for patients with AML ineligible for intensive chemotherapy (IC) due to advanced age ( >75 years) or significant comorbidities based on improved outcomes compared to HMA alone [2]. In the VIALE-A randomized trial, administering AZA at 75 mg/m²/day for 7 days every 28 days alongside once-daily VEN at 400 mg demonstrated significant improvement in composite complete remission (CRc) rates (66.4% vs. 28.3%) and overall survival (OS) (14.7 vs. 9.6 months) compared to AZA alone. These benefits were observed across various patient subgroups, particularly among those harboring NPM1 or IDH2 somatic mutations [3, 4]. Despite the recommendation for continuous VEN, VEN interruptions followed by VEN duration reduction to 21 days after leukemia clearance from bone marrow to allow hematologic recovery is necessary in more than 70% of responders during the consolidation phase, and does not appear to negatively affect OS [2, 5, 6]. In alignment with these commonly performed VEN duration modifications to improve tolerability, the European LeukemiaNet (ELN) recommends considering a reduction in the duration of VEN therapy from 28 to 21 or even 14 days (or less) in responders experiencing delayed count recovery or recurrent grade 4 neutropenia/thrombocytopenia lasting ≥ 7 days [1]. It is noteworthy that various durations of VEN therapy were not assessed in the original dose-escalation studies for AML, underscoring that optimal VEN duration to maximize efficacy without exacerbating toxicity remains unknown. In this context, we retrospectively analyzed a multicentric cohort of patients with newly diagnosed (ND) AML and severe comorbidities precluding IC who received AZA alongside a shortened VEN duration (administered only during the 7 days of AZA beginning with the first cycle) and compared it to a large cohort of patients who received HMA with the standard VEN duration.
Methods
Patients and treatment
Eighty-two off-protocol IC-ineligible patients from 7 French centers with ND AML who received at least one cycle of AZA and concomitant once daily VEN for 7 days from the first cycle (“7 + 7” cohort) between July 2019 and March 2022 were included in the retrospective analysis. Reasons for a short VEN exposure were determined by the treating physician in each center and included exclusion criteria to the standard VEN scheme established in the VIALE-A protocol due to more severe comorbidities than allowable on study (as concurrent malignancy). All patients in the “7 + 7” cohort received a first cycle of subcutaneous AZA 75 mg/m²/day for 7 days associated with once daily ramp-up of venetoclax for 7 days (100 mg at day 1, 200 mg at day 2, 400 mg from day 3 to 7 in case of no concomitant azole) and were managed in a out- or in-patient manner with tumor lysis syndrome (TLS) prophylaxis. VEN dose was adjusted based on drug interactions, particularly with azole antifungal prophylaxis. Bone marrow (BM) aspiration was obtained after cycle 1 or 2 based on treating physician discretion. Minimal residual disease (MRD) assessment by flow cytometry was performed in a subset of patients. Treatment cycle delays/interruption, dose reductions, as well as G-CSF use were determined by the treating physician from each center.
Results were compared to a historical cohort of US patients (N = 166) from MD Anderson Cancer Center who received HMA (AZA 75 mg/m²/day for 7 days; intravenous decitabine (DAC) 20 mg/m²/d for 5 or 10 days; oral ASTX727 35 mg/100 mg for 5 days) associated with standard dose and duration of VEN (“std-HMA/VEN” cohort). Standard dose and duration of VEN was defined as the physician intent for at least 14 days or more of venetoclax during cycle 1.
Disease characteristics and mutational status
BM analysis including conventional karyotype and molecular status using next-generation sequencing (NGS) were performed locally and retrospectively reviewed. Mutation status was uniformly assessed for 28 genes (NPM1, FLT3 (ITD/TKD), CEBPA, RUNX1, ASXL1, TP53, IDH1, IDH2, BCOR, CBL, CSF3R, DNMT3A, EZH2, GATA2, JAK2, MPL, PHF6, PTPN11, SF3B1, KRAS, NRAS, SH2B3, SRSF2, STAG2, TET2, U2AF1 and ZRSR2). Minimum reportable variant allele frequency (VAF) was 5% of sequencing depth of at least 100X. Disease risk was classified according to the 2017 ELN risk stratification and additionally the molecular prognostic risk signature (mPRS) using N/KRAS, FLT3-ITD, and TP53 mutations to stratify patients into three groups (higher, intermediate, and lower benefit) [7,8,9].
Outcomes
Disease assessments were performed using the modified International Working Group response criteria for AML [10]. Complete remission (CR) was defined as an absolute neutrophil count of more than 1000 cells per cubic millimeter, a platelet count of more than 100 000 per cubic millimeter and bone marrow with less than 5% blasts. Complete remission with incomplete hematologic recovery (CRi) was defined as all the criteria for complete remission, except for residual neutropenia (absolute neutrophil count, ≤1000/mm3) or thrombocytopenia (platelet count, ≤100 G/L). Composite complete remission (CRc) was defined as CR or CRi. Progressive disease was defined according to the ELN recommendations. Platelet transfusion policy was identical in all centers, i.e. if the platelet count ≤20 G/L or if thrombocytopenia with significant bleeding. Platelet transfusion independence was defined as the absence of platelet transfusion for at least 56 days between the first and last day of treatment. In patients who had composite complete remission, measurable residual disease (MRD) was assessed by flow cytometry, with negativity defined according to ELN guidelines as aberrant phenotypic blasts <0.1% of BM cells in an adequate sample [1].
Overall survival (OS) was defined as the number of days from cycle 1 day 1 to the date of death from any cause; event-free survival (EFS) was defined as the number of days from cycle 1 day 1 to disease progression, treatment failure (failure to achieve complete remission or <5% bone marrow blasts after at least 6 cycles of treatment), confirmed relapse, or death. Non-responding patients were considered to have an EFS event on day 1. Data for each patient were censored at the date of the last visit or the date on which the patient was last known to be alive.
Statistical analysis
Patient characteristics were summarized by frequency (percentage) for categorical variables and median (range) values for continuous variables. The statistical significance in differences between categorical variables was determined using the Chi-square or Fisher’s exact test. Differences between continuous variables were evaluated using the Wilcoxon Mann-Whitney test. OS and EFS were estimated using the Kaplan-Meier method and differences between groups evaluated using the log-rank test. Multivariate analyses were performed using Cox proportional hazards regression. The proportional hazard assumption was checked with the Schoenfeld residuals.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. The present study was approved by the institutional review board (Health data hub AZAVENADAPT N° F20220802094251; Institutional Review Board N°2022-124). Informed consent was obtained in all cases according to the Declaration of Helsinki.
Results
Characteristics of the cohorts
The “7 + 7” cohort (n = 82) was enriched in patients who had significant comorbidities defined as exclusion criteria to clinical trials. Reported reasons to reduce VEN duration prior to treatment initiation by treating physicians included: anticipated toxicity of a standard VEN exposure due to frailty, number and severity of comorbidities, adverse risk disease, and often all these reasons combined. Retrospective evaluation captured that 36 patients (44%) had at least 1 exclusion criteria to the VIALE-A study protocol (Supplemental Table 1). The main reported exclusion criteria was previous cancer treated with a non-curative intent (i.e. without surgical resection) or a concomitant cancer (i.e. metastatic, in situ disease, or cancer on active therapy). Furthermore, at least 13 supplemental patients (16%) were declared as significantly frail due to significant medical comorbidities among patients who could fulfill VIALE-A inclusion criteria. Among patients younger than 75 years (N = 35), 14 patients (40%) had a cancer treated with non-curative intent. Five patients younger than 60 years were treated with the “7 + 7” scheme due to concomitant metastatic solid cancer (n = 2; thymic carcinoma, non-seminomatous germ cell cancer), severe cardiomyopathy (n = 1) and severe malnutrition related to previous medical condition (n = 2). Conversely, 142/166 (86%) subjects from “std-HMA/VEN” cohort received treatment in the context of a clinical trial.
The baseline patient characteristics of the “7 + 7” and “std-HMA/VEN” cohorts are presented in Table 1. Except for comorbidities, characteristics were mostly balanced between cohorts with a median age of 75 and 74 years old, an altered ECOG performance status (PS = 2–4) in 37% and 32%, diploid cytogenetic in 43% and 36%, and adverse ELN 2017 in 64% and 67% respectively.
Secondary AML to a prior MDS or MPN (32% vs 18%), post-cytotoxic therapy AML (34% vs 22%) and FLT3-ITD mutations (13% vs 2%) were enriched in “7 + 7” cohort, while complex cytogenetics (22% vs 39%) and N/KRAS mutations (9% vs 24%) were more prevalent in “std-HMA/VEN” patients. TP53, NPM1 and IDH1/2 mutation rates were generally similar between cohorts but a higher proportion of patients from the “7 + 7” cohort had a predicted higher benefit with HMA-VEN defined by mPRS (58% vs 41% in “std-HMA/VEN” cohort). This discrepancy was attenuated by a significant higher rate of NPM1 mutation (which is known to confer a greater sensitivity to HMA-VEN combination) within mPRS high-benefit patient from “std-HMA/VEN” cohort (30% vs 11% in “7 + 7” cohort; P = 0.017) and a trend for a lower frequency of 17p abnormalities (0% vs 7% respectively; P = 0.081). (Supplemental Table 2).
Treatment modalities between cohorts
Among patients from “7 + 7” cohort, 22 patients (34%) received hydroxyurea before starting Venetoclax. Azole antifungal use is summarized in Supplemental Table 3. During VEN ramp-up in cycle 1, target dose was 400 mg/d in 49 patients (60%) and ≤200 mg/d in 33 patients (including 29 due to concomitant posaconazole). After cycle 1 and/or cycle 2, 58% of CR/CRi patients (34/59) received G-CSF to limit grade III/IV neutropenia duration. Supplemental Figure 1 presents treatment flow-chart of the “7 + 7” cohort. Twenty-six percent of patients discontinued treatment after 1 or 2 cycles and 26% were still on therapy at data cut-off. The main reasons for discontinuing therapy were lack of response or relapse (61%), or toxicity while in CR/CRi/MLFS (29%). Of note, only 1 patient in stable disease after cycle 1 received a more extended duration of VEN during the second cycle and AML disease remained refractory after the second cycle. Among patients who obtained CRc, 66% had further dose and/or delay adjustment mainly due to cytopenias. For example, 95% had subsequent cycles decreased to 5 days (AZA with VEN 400 mg daily for 5 days) after a median of 3 cycles (range: 2-7), 69% had a reduced VEN dose of 200 mg/d (in absence of CYP3A4 inducer) after a median of 4 cycles (range: 2-18) while a 5 weeks interval between cycles was applied in 46% of cases after a median of 5 cycles (range: 3-25). Consequently, 15 CRc patients (25%) were then treated with cycles composed of 5 days of AZA concomitantly to VEN 200 mg/d every 5 weeks.
Treatment modalities from “std-HMA/VEN” cohort are presented in Supplemental Figure 2. The most common cycle 1 HMA regimen was intravenous DAC for 10 days (60% of patients) and the most common cycle 1 VEN duration was >21 days (67%). During cycles 2 and 3, the most common DAC duration was 5 days (71% of patients in cycle 2, 79% in cycle 3) while VEN duration remained >14 days in the majority of patients (68% in cycle 2, 60% in cycle 3). A few patients (7%) from the “std-HMA/VEN” cohort only received 7 or 14 days of VEN during cycle 1 due to unplanned complication. These patients were retained in the cohort per the “intention to treat” principle.
Efficacy and outcomes
Patients treated with a 7-day regimen received significantly more cycles than “std-HMA/VEN” (median number of cycles: 6 vs 3 respectively; P < 0.01). Conversely, the median time between the first 3 cycles were lowered in “7 + 7” cohort compared to “std-HMA/VEN” cohort (30 vs 39 days between cycle 1 and 2, 28 vs 36 days between cycle 2 and cycle 3, respectively).
The overall responses rates between cohorts were compared (Table 2). The overall CRc rate with the “7 + 7” scheme (72%) was not significantly different from that of “std-HMA/VEN” (72%; P = 0.955) as well as rates of negative MRD by flow cytometry (70% vs 53% respectively; P = 0.058). There was also no difference in CR rate with complete recovery in “7 + 7” cohort when compared to “std-HMA/VEN” patients. Exploratory subgroup analyses for CRc rate showed no differences according to IDH1/2 (93% vs 84%; P = 0.648), NPM1 (92% vs 90%; P = 1.000) or TP53 mutation status (50% vs 61%; P = 0.445) between “7 + 7” and “std-HMA/VEN” cohorts respectively (Supplemental Table 4).
Time to response was delayed in patients who received a 7-day VEN scheme. Median cycles to first response was 1 in both groups but 42% of responders on “7 + 7” required more than 1 cycle for first response, whereas almost all responders on “std-HMA/VEN” (99%) had a first response after cycle 1 (Fig. 1A). As shown by Fig. 1B, “7 + 7” patients required an increased number of cycles to obtain best response (2 vs 1 in standard cohort; P = 0.03). However, this observation should be mitigated as 15/82 (18.3%) of patients from “7 + 7” cohort didn’t have BM evaluation after cycle 1.
At a median follow-up of 19.4 months in the “7 + 7” cohort and 34.7 months in the “std-HMA/VEN” cohort, median OS was not significantly different between cohorts (11.2 months vs 10.3 months, respectively; P = 0.75) (Fig. 2A). Two-year OS was 28% vs 34% for “7 + 7” vs “std-HMA/VEN” respectively. Analysis for EFS showed similar results without significant difference between “7 + 7” (median EFS: 6.5 months) and “std-HMA/VEN” cohorts (median EFS: 7.4 months; P = 0.66) (Fig. 2B). Two-year EFS was 25% vs 27% for “7 + 7” vs “std-HMA/VEN” respectively. Due to comorbid condition of patients from “7 + 7” cohort, a significant larger proportion of patients from “std-HMA/VEN” cohort transitioned to allogeneic stem-cell transplantation (SCT) (14% vs 1% in “7 + 7” cohort; P = 0.001). However, there was no difference when survival analysis were censored for allogeneic SCT (Supplemental Figure 3). OS and EFS also remained similar when the “7 + 7” cohort was compared to patients who received DAC for 10 days (N = 102), regardless of TP53 mutation status (Supplemental Figure 4).
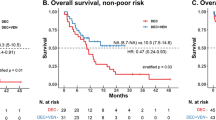
To better assess if a subset of patients could have improved OS with either “7 + 7” or “std-HMA/VEN”, we stratified patients according to mPRS when TP53, N/KRAS and FLT3-ITD mutation status were available (N = 80 for “7 + 7”; N = 135 for “std-HMA/VEN”) (Fig. 3). In that setting, VEN duration did not influence OS from TP53 mutant AML patients (low benefit group; median OS: 4.6 vs 5.2 months in “7 + 7” vs “std-HMA/VEN” respectively; P = 0.466) as well as OS from patients with FLT3-ITD or N/KRAS mutation (intermediate benefit group; median OS: 8.4 vs 12.4 months respectively; P = 0.971). However, patients with a high predicted benefit from HMA-VEN regimen had a decreased OS after 12 months with the “7 + 7” regimen (median OS: 14.1 vs 32.0 months in “7 + 7” and “std-HMA/VEN”; P = 0.046). This difference was no longer statistically significant when patients were censored at allogeneic SCT (median OS: 14.1 vs 29.7 months in “7 + 7” and “std-HMA/VEN”; P = 0.08) (Supplemental Figure 5). The same tendency was observed in patients with a high-predicted benefit defined by mPRS with an IDH1/2 mutation (P = 0.056) (Supplemental Figure 6).
A Overall survival stratified by treatment cohort and molecular prognostic risk signature (mPRS) predicted benefit (high, intermediate, or low benefit). B Event-free survival stratified by treatment cohort and mPRS. EFS event-free survival, HMA hypomethylating agent, mPRS molecular prognostic risk signature, OS overall survival, SCT stem cell transplant, VEN venetoclax.
We therefore performed univariate and multivariate analysis to overcome multiple confounding factors that could influence OS. In the whole cohort, ECOG PS ≥ 2 and mPRS intermediate benefit were the only significant predictive factors for inferior OS, while presence of NPM1 mutation was the only independent factor for a higher OS (Fig. 4A). In the whole cohort, treatment setting (i.e. “7 + 7” or “st-HMA/VEN”) had no independent impact on OS. To clarify the impact of VEN duration in patients who benefit most from treatment, we also applied multivariate analysis on cohort with high-predicted benefit defined by mPRS (Fig. 4B). In that cohort, only ECOG PS ≥ 2, complex cytogenetic and AML arising from MDS/MPN were negative independent predictors of OS while treatment scheme (i.e. “7 + 7” or “st-HMA/VEN”) had no independent impact on OS. In addition, there was no variable with independent impact on survival in patients with intermediate and lower benefit by mPRS, including ECOG PS ≥ 2 (Supplemental Figure 7).
A Univariate and multivariate analysis of overall survival in whole cohort. B Univariate and multivariate analysis of overall survival in patients with a high-benefit predicted by molecular prognostic risk signature (mPRS). AML-pCT AML post-cytotoxic therapy, CG cytogenetic, DAC decitabine, HMA hypomethylating agent, mPRS molecular prognostic risk signature, OS overall survival, PS Performance Status, VEN venetoclax.
Hematological toxicities and early mortality
As shown in Table 3, a 7-day VEN duration does not appear to strongly modify early hematological toxicity. Indeed, patients from “7 + 7” and “std-HMA/VEN” cohorts had similar febrile neutropenia rates (48% vs 55% during cycle 1 respectively; P = 0.28) as well as red-blood-cell transfusion (RBC) requirements (84% vs. 83% during cycle 1 respectively; P = 0.71). However, a significant reduction in platelet transfusion requirement was observed in “7 + 7” cohort during cycle 1 (62% vs. 77% in “std-HMA/VEN”; P = 0.02). During subsequent cycles in “7 + 7” cohort, toxicity remained sufficiently significant by local physician to further reduce the scheme in 58% of responders. Beginning with cycle 3 and beyond, 73% of “7 + 7” patients didn’t require any additional platelet transfusions and 62% remained free of any additional cases of febrile neutropenia or grade III/IV infection.
We also evaluated if a reduced VEN exposure could influence early mortality rate. Early death rate at 4 weeks after treatment initiation were similar (2% vs. 5% respectively) between cohorts but 8-week mortality was significantly reduced in “7 + 7” cohort when compared to “std-HMA/VEN” (6% vs. 16% respectively; P = 0.03). Of note, 45% (14/31) of the 8-week mortality occurred in TP53-mutated patients. No early death occurs in responding patient from “7 + 7” cohort (4/5 died from sepsis) while only 2/26 deaths occurs in responders from “std-HMA-VEN” cohort (death from cardiac failure).
Discussion
HMA-VEN combination therapies represent a significant advance in treating patients with AML who are ineligible for IC [2, 11]. However, despite their effectiveness, these regimens are hindered by considerable toxicity, often necessitating VEN treatment interruption, delays in HMA-VEN cycles or even discontinuation of therapy due to cytopenias and cytopenia-related complications. Recent updates from the VIALE-A trial revealed that 76% of responders had a median VEN duration of ≤21 days during cycles, without any signal for inferior outcomes following this dose reduction [6]. Although early-phase clinical trials testing VEN-HMA combinations in AML have established the maximum tolerated dose of VEN, the optimal VEN duration during cycles was never investigated and remains undetermined [12, 13]. Continuous VEN administration has been arbitrarily defined based on the original phase II clinical trial testing VEN as a single agent in relapsed and refractory AML patients, as well as the experience of VEN monotherapy for chronic lymphocytic leukemia [14]. In the R/R AML setting, continuous VEN monotherapy had only modest and non-durable activity, as compared to HMA-VEN combination therapy, highlighting the importance of VEN-HMA synergy [15]. Consequently, the efficacy of a prolonged VEN exposure as a single agent following AZA administration remains uncertain. To answer that question, a recent retrospective study conducted by the Mayo Clinic compared outcomes among patients who received AZA combined with VEN exposure for 28, 21, or 14 days during cycles [16]. Results suggest that a 14-day VEN administration may be as effective as standard VEN exposure in terms of response or survival, although again without a clear reduction in cytopenia and infection rates. Our study aimed to investigate whether VEN intake limited to the 7 days of AZA administration from the first cycle could be as effective as standard VEN exposure, through a retrospective comparison with patients who received standard VEN exposure.
Our study is subject to several limitations related to the differences between cohorts. Firstly, its retrospective nature prevented the capture of frailty and comorbidities that could potentially influence both toxicity and long-term outcomes. Even if ECOG PS was similar between cohorts, almost half of “7 + 7” cohort presented at least 1 exclusion criterion upon entry into a clinical trial, unlike the vast majority of the “std-HMA/VEN” cohort. This could reflect differences in patient’s fitness that we were not able to capture adequately. The difference in HMA use between cohorts could also limit the interpretation of the results since the majority of the “std-HMA/VEN” cohort received DAC for 10 days, a potentially more toxic regimen than AZA for 7 days. Concerning AML characteristics between cohorts, AML arising from a prior MDS or MPN and AML post-cytotoxic therapy were enriched in “7 + 7” cohort and complex cytogenetics more frequent in “std-HMA/VEN” cohort, but TP53 mutation rates were yet comparable. Likewise, “7 + 7” cohort presented a higher rate of FLT3-ITD mutation while the comparative cohort had increased N/K-RAS mutation rate (FLT3-ITD patients were prioritized to FLT3 inhibitor-based regimens) but these mutations had similar prognostic impact on mPRS stratification. Ultimately, “7 + 7” cohort was enriched in patient with a high-predicted benefit defined by mPRS but that difference is mitigated by a higher rate of NPM1 mutation within mPRS high-benefit patient from “std-HMA/VEN” cohort and a trend for a lower frequency of 17p abnormalities.
Despite these limitations, we did not observe a difference in response rates (CRc rate: 72% vs 72% respectively; negative phenotypic MRD in responders: 70% vs 53% respectively) or overall survival (median OS: 11.2 vs 10.3 months respectively) between a 7-day course of VEN and standard exposure in newly diagnosed AML patients. These findings align with real-life registries where median overall survival was inferior to VIALE-A phase III results [17,18,19,20]. Of note, our study also demonstrated that mPRS is a powerful tool to stratify survival in AML patients treated with less-intensive therapies.
Although overall the 7-day VEN regimen compares favorably to the control cohort, trend for a reduced survival in patients with a better prognosis defined by baseline mutations, especially patients with IDH1/2 mutation, suggests that a subset of patients may benefit most from a prolonged VEN duration. This observation would be consistent with the known sensitivity of IDH1/2-mutant AML cells to Bcl-2 inhibition and the clinical response observed in IDH1/2-mutant AML patients treated with VEN monotherapy [14, 21]. This trend in the long-term survival of the highest mPRS “7 + 7” cohort may nevertheless have been influenced by further dose reductions in the consolidation phase, shorter follow-up, severe comorbidities that initially motivated physician to arbitrarily reduce VEN duration or both. Finally, multivariate analysis of survival reveals that VEN reduced to 7 days was not detrimental in patients with a high-benefit defined by mPRS. Despite these encouraging results, our retrospective comparison does not allow to definitely conclude on the non-inferiority of a 7-day scheme and caution should be exercised regarding patients with a high mPRS benefit (i.e. without FLT3-ITD, N/KRAS or TP53 mutation) [8, 9, 18]. Of note, although mPRS stratification represents a stronger prognostic tool than 2022 ELN risk classification in patients receiving less-intensive therapies, outcomes remain heterogeneous in the high-benefit mPRS group. Our study shows that complex cytogenetic, even rare in that subgroup, had a high independent negative prognostic value. This result highlights the potential role of other genetic characteristics in better defining prognosis within the high-benefit mPRS group.
Previous retrospective studies suggests that reducing VEN duration might be less toxic [16, 22]. Our current study suggests that early hematological toxicity during the first cycles appears inevitable regardless of VEN exposure duration and is likely due to the profound synergy seen during the first week of treatment. A 7-day VEN exposure was associated with a lower platelet transfusion burden during cycle 1, although this difference may be partially explained by a higher platelet baseline count in “7 + 7” cohort. A 7-day VEN exposure was also associated with the shorter delay between the first 3 cycles, which could indirectly argue in favor of better hematological recovery. More importantly, a 7-day VEN regimen was associated with a reduction in early mortality at 8 weeks (6%) in a particularly frail population. Such results compare favorably to the control cohort and already published results from a real life registry [19].
Altogether, our study underscores the potential efficacy and practical advantages of reducing VEN course to 7-day during HMA-VEN therapy from the first cycle for ND AML patients. Future studies will be needed to confirm these datas but we encouragingly identified similar responses and minimal early mortality in older and frail patients with the use of a “7 + 7” scheme. Importantly, this approach offers several practical benefits, including improved drug compliance, mitigation of potential drug interactions (especially in older patients with multiple comorbidities requiring numerous medications), healthcare cost reduction and increased confidence among physicians regarding the use of VEN-HMA combination therapy in very frail patients. Furthermore, our findings may have implications for patients with myelodysplastic syndrome, where clinical trials investigating AZA-VEN combinations have necessitated VEN exposure reduction. Finally, our results could inform future clinical trials exploring triplet combination therapies in first-line AML treatment, where VEN exposure reduction has already been implemented during both induction and consolidation phases [23,24,25,26,27]. However, caution remains warranted (especially in patients with “venetoclax-sensitive” genomics) and studies are needed to definitively address the question of VEN duration in unfit AML patients; a prospective phase III trial randomizing the “7 + 7” scheme with standard VEN exposure is planned (SEVENAZA trial) [28].
Data availability
The data used for this study are not publicly available to protect patient confidentiality. Deidentified data are available on reasonable request from the corresponding author (stephane.debotton@gustaveroussy.fr).
References
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl J Med. 2020;383:617–29.
DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791–803.
Pollyea DA, DiNardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, et al. Impact of Venetoclax and Azacitidine in Treatment-Naïve Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin Cancer Res. 2022;28:2753–61.
Pratz KW, DiNardo CD, Selleslag D, Li J, Yamamoto K, Konopleva M, et al. Postremission cytopenia management in patients with acute myeloid leukemia treated with venetoclax and azacitidine in VIALE‐A. Am J Hematol. 2022;97:E416–E419.
Pratz KW, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Döhner H, et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am J Hematol. 2024;99:615–24.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Döhner H, Pratz KW, Dinardo CD, Jonas BA, Pullarkat VA, Thirman MJ, et al. ELN Risk Stratification Is Not Predictive of Outcomes for Treatment-Naïve Patients with Acute Myeloid Leukemia Treated with Venetoclax and Azacitidine. Blood. 2022;140:1441–4.
Döhner H, Dinardo CD, Jonas B. Genetic Risk Stratification and Outcomes Among Treatment-Naive Patients With AML Treated With Venetoclax and Azacitidine. Blood. 2024;44:2211–22
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised Recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9.
DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7:e724–36.
DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–28.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute Myelogenous Leukemia. Cancer Discov. 2016;6:1106–17.
Jin S, Cojocari D, Purkal JJ, Popovic R, Talaty NN, Xiao Y, et al. 5-azacitidine induces NOXA to prime AML cells for venetoclax-mediated apoptosis. Clin Cancer Res. 2020;26:3371–83.
Karrar O, Abdelmagid M, Rana M, Iftikhar M, McCullough K, Al-Kali A, et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: Comparative analysis of response, toxicity, and survival. Am J Hematol John Wiley Sons Inc. 2024;99:E63–6.
Gangat N, Karrar O, Iftikhar M, McCullough K, Johnson IM, Abdelmagid M, et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: Genotype signatures for response and survival among 301 consecutive patients. Am J Hematol. 2024;99:193–202.
Bataller A, Bazinet A, DiNardo CD, Maiti A, Borthakur G, Daver NG, et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 2024;8:927–35.
Matthews AH, Perl AE, Luger SM, Loren AW, Gill SI, Porter DL, et al. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022;6:3997–4005.
Todisco E, Papayannidis C, Fracchiolla N, Petracci E, Zingaretti C, Vetro C, et al. AVALON: The Italian cohort study on real‐life efficacy of hypomethylating agents plus venetoclax in newly diagnosed or relapsed/refractory patients with acute myeloid leukemia. Cancer. 2023;129:992–1004.
Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–84.
Aiba M, Shigematsu A, Suzuki T, Miyagishima T. Shorter duration of venetoclax administration to 14 days has same efficacy and better safety profile in treatment of acute myeloid leukemia. Ann Hematol. 2023;102:541–6.
Short NJ, Daver N, Dinardo CD, Kadia T, Nasr LF, MacAron W, et al. Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3 -Mutated AML. J Clin Oncol. 2024;42:1499–508.
Yilmaz M, Muftuoglu M, Dinardo CD, Kadia TM, Konopleva MY, Borthakur G, et al. Phase I/II Study of Quizartinib, Venetoclax, and Decitabine Triple Combination in FLT3-ITD Mutated AML [Internet]. Blood. 2023;142:158–61.
Lachowiez CA, Loghavi S, Zeng Z, Tanaka T, Kim YJ, Uryu H, et al. A Phase Ib/II Study of Ivosidenib with Venetoclax ± Azacitidine in IDH1-Mutated Myeloid Malignancies. Blood Cancer Discov. 2023;4:276–93.
Issa GC, Cuglievan B, DiNardo CD, Short NJ, McCall D, Gibson A, et al. Early Results of the Phase I/II Study Investigating the All-Oral Combination of the Menin Inhibitor Revumenib (SNDX-5613) with Decitabine/Cedazuridine (ASTX727) and Venetoclax in Acute Myeloid Leukemia (SAVE). Blood Am Soc Hematol. 2023;142:58–58.
Lane AA, Garcia JS, Raulston EG, Garzon JL, Galinsky I, Baxter EW, et al. Phase 1b trial of tagraxofusp in combination with azacitidine with or without venetoclax in acute myeloid leukemia. Blood Adv. 2024;8:591–602.
Döhner H, Niederwieser D, Röllig C, Sierra Hospital Santa Creu Sant Pau J, Eytan Stein Memorial Sloan S. Genetic risk classification for adults with AML receiving less-intensive therapies: the 2024 ELN recommendations. Blood. 2024;144:2169–73.
Acknowledgements
We thank Veronique Saada, Veronique Verge and Ahmadreza Arbab for cytological analysis, Nathalie Auger for conventional cytogenetics.
Author information
Authors and Affiliations
Contributions
CW, ABaz, SC, ABat, JD, NA, BC, CR, DL, AM, NG, NS, SB, KS, SKH, MS, JBM, FP, DRW, LP, ND, TK, DB, FR, HK, SDB and CD followed study patients. CM supervised and performed molecular analysis; CW and ABaz collected data and interpreted the results; AP, ABaz and ABat performed statistical analysis; CW wrote the manuscript; CD and SDB supervised the project; ABaz, CD and SDB revised the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
CW: Consultant/Advisory Boards: BMS, Abbvie. NG: Consultant/Advisory Boards: BMS. JBM: Honoraria: Jazz Pharmaceuticals, Astellas Pharma, SERVIER. Consultant/Advisory Boards: AbbVie, Gilead Sciences. Travel, Accommodations, Expenses: AbbVie. CM: Research funding: Incyte. LP: Consultant/Advisory Boards: Janssen, Takeda, Abbvie, Gilead, Kephren and Résilience. SDB: Consultant/Advisory Boards: Servier, BMS, GSK, Syndax Pharmaceuticals, and Remix Therapeutics. Honoraria from BMS, AbbVie, Servier, Jazz Pharmaceuticals, Astellas, and Loxo Oncology. Speakers’ bureau: Servier, BMS, Jazz Pharmaceuticals, Astellas, and AbbVie. Research funding: Forma Therapeutics and Auron Therapeutics. CD: Consultant/Advisory Boards: Abbvie, AstraZeneca, Astellas, BMS, Genentech, GenMab, GSK, ImmuneOnc, Notable Labs, Rigel, Schrodinger, Servier. CDD is supported by the LLS Scholar in Clinical Research Award. ABazinet, SC, ABataller, JD, NA, BC, CR, DL, AM, NS, SB, KS, SKH, MS, FP, DRW, ND, TK, DB, FR, AP and HK had no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Willekens, C., Bazinet, A., Chraibi, S. et al. Reduced venetoclax exposure to 7 days vs standard exposure with hypomethylating agents in newly diagnosed AML patients. Blood Cancer J. 15, 68 (2025). https://doi.org/10.1038/s41408-025-01269-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-025-01269-x
This article is cited by
-
Comprehensive view on chemotherapy-free management of acute myeloid leukemia by using venetoclax in combination with targeted and/or immune therapies
Cell Death Discovery (2025)
-
Newly diagnosed acute myeloid leukemia in unfit patients: 2026 treatment algorithms
Blood Cancer Journal (2025)
-
Comparable outcomes with 14-, 21-, or standard 28-day venetoclax in the first cycle of azacitidine–venetoclax in untreated acute myeloid leukemia: real-world experience from the Hokkaido Leukemia Net
Blood Cancer Journal (2025)